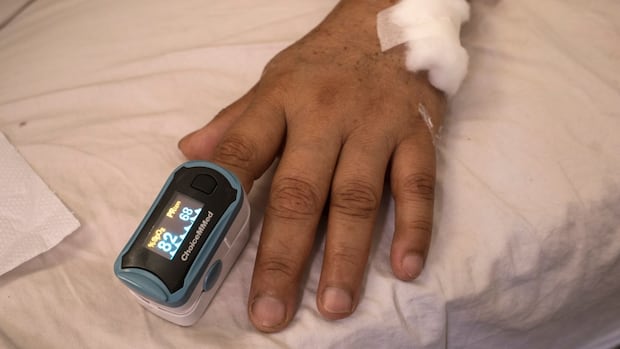
The U.S. Food and Drug Administration proposed new guidance for devices used to monitor blood oxygen levels to improve their accuracy and performance across a range of skin tones.
FDA says recommendations address accuracy concerns of medical devices affected by factors like skin tone
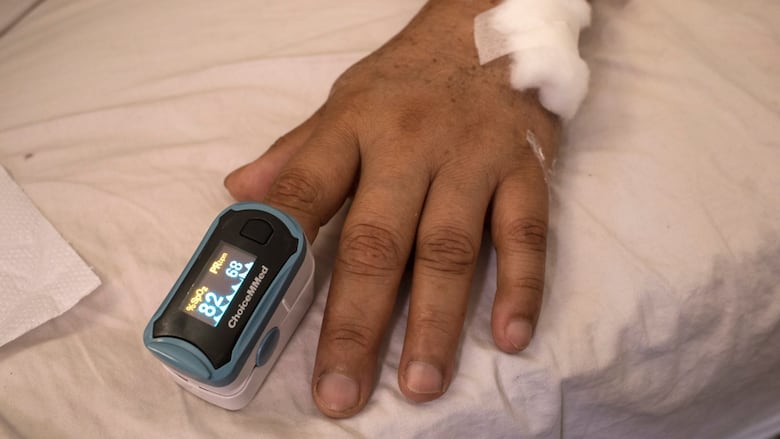
The U.S. Food and Drug Administration on Monday proposed new guidance for devices used to monitor blood oxygen levels to improve their accuracy and performance across a range of skin tones.
The updated guidance recommends that manufacturers gather clinical data to evaluate device performance accuracy across a range of skin pigmentations and increase the number of participants on whom these devices are tested.
“Our draft recommendations are based on the best available science to help address concerns of disparate performance of pulse oximeters based on an individual’s skin pigmentation,” said Michelle Tarver, director of the FDA’s Center for Devices and Radiological Health.
The regulator said the guidance, if finalized, would apply to oxygen monitoring devices known as pulse oximeters that are used for medical purposes.
It would not apply to the devices that are sold for general wellness and are not reviewed by the agency.



