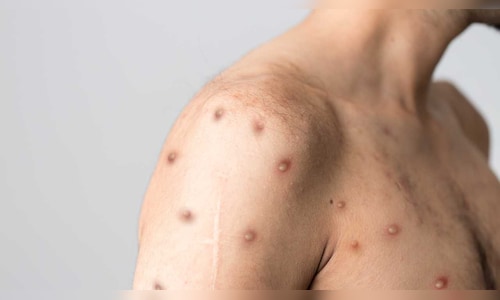
The World Health Organisation (WHO) declared mpox as a Public Health Emergency of International Concern (PHEIC) in August this year. Mpox is a self-limiting viral infection that can cause severe infections in certain groups including, malnourished children, pregnant women, and immunocompromised persons.
Reports indicate that three mpox epidemics are presently occurring, each with a different viral variant. A worrying trend is more than half of Congo’s mpox cases this year affected children and globally, out of all the people who have succumbed to the illness, 80% of them were under the age of 15. Infections in children is likely due to underlying malnourishment or compromised immunity, primarily since many children grow up in areas of conflict and displacement, and are already under the threat of other diseases, such as polio and cholera.
Increased urbanisation, population growth, and bushmeat consumption place people in closer contact with rodents and are drivers of zoonotic spillover events, and the increased incidence of outbreaks.
Emergence of periodic mpox outbreaks has been linked to waning immunity against smallpox. Vaccinations against smallpox provides a certain degree of immune protection against mpox. Researchers theorise that since vaccination campaigns against smallpox ceased over 30 years ago, a considerable part of the population no longer possesses immunity towards smallpox (and mpox) and herd immunity is thus on the decline.
The
mpox vaccines that are currently approved by regulatory agencies — JYNNEOS and ACAM2000 — are considered as safer third-generation vaccines that elicit protective immune responses. A third approved vaccine — LC16m8 — is not advisable for pregnant women and immunocompromised persons, limiting its scope.
Over 275,000 vaccine doses arrived in Africa earlier this month. Reports indicate that 10 million doses are necessary to control the current outbreak but only half of this has been pledged by donor countries thus far. To address the current dilemma, the WHO’s Expression of Interest in Emergency Use Listing has set in motion mechanisms to trigger GAVI and UNICEF to procure vaccines and diagnostics for distribution.
The recent decision by Indian government to formulate high-performance biomanufacturing policies under BioE3 and Bio-RIDE are envisioned to promote biotechnology for economy, environment, and employment, and cutting-edge research and development. The policies are set to attract ₹10,000 crore investments. Strategic implementation of the manufacturing of vaccines and diagnostics under these policies can boost India’s bioeconomy, which has grown exponentially in the last ten years by $120 billion.
India reported two mpox cases since PHEIC was declared but its risk of spread is said to be low. India had reported its last case in March earlier this year and had 30 confirmed cases in the 2022 mpox outbreak indicating that domestic production of mpox vaccines would serve India’s health security measures. India’s top vaccine maker Serum Institute of India (SII) has expressed its plans to work with Novavax to produce mRNA vaccines to combat mpox.
Steps could be initiated to produce and distribute mpox vaccines to Africa as part of India’s vaccine diplomacy initiatives. The availability of diagnostics is also of great concern, and is essential to adequate healthcare. India’s Central Drugs Standard Control Organisation (CDSCO) recently approved the manufacturing of domestically developed mpox diagnostic kits that have been validated by ICMR. This is a significant stride to building resilience during the current PHEIC and a chance address the demand for diagnostics.
—The author, Lakshmy Ramakrishnan, is Associate Fellow at Observer Research Foundation. The views expressed are personal.
(Edited by : Unnikrishnan)



