
Three new medicines recommended for approval
EMA’s human medicines committee (CHMP) recommended three medicines for approval at its January 2024 meeting.
The committee recommended granting a marketing authorisation for Exblifep (cefepime/enmetazobactam), for the treatment of complicated urinary tract infections, including pyelonephritis, hospital-acquired pneumonia, including ventilator associated pneumonia, and for the treatment of patients with bacteraemia (the presence of bacteria in the bloodstream) caused by the infections listed above.
Ryzneuta (efbemalenograstim alfa) received a positive opinion from the CHMP for the reduction in the duration of neutropenia (low levels of neutrophils, a type of white blood cell) and the incidence of febrile neutropenia due to chemotherapy.
The CHMP also gave a positive opinion to Niapelf (paliperidone), a generic medicine for the treatment of schizophrenia.
Negative opinion for two medicines
The CHMP recommended the refusal of marketing authorisations for Nezglyal* (leriglitazone), intended for the treatment of paediatric and adult male patients aged two years and older with cerebral adrenoleukodystrophy, a genetic condition that damages the membrane that covers nerve cells in the brain and spinal cord, and Syfovre (pegcetacoplan), for the treatment of geographic atrophy secondary to age-related macular degeneration, a progressive retinal macular disease causing gradual vision impairment mainly in the elderly.
For more information on these negative opinions, see the question-and-answer documents in the grid below.
Recommendations on extensions of therapeutic indication for four medicines
The committee recommended extensions of indication for four medicines that are already authorised in the European Union (EU): Abecma*, Aspaveli*, Prevenar 20 (previously Apexxnar), and Retsevmo.
Outcome of re-examination
Following a re-examination, the CHMP confirmed its original recommendation to not renew the conditional marketing authorisation for Translarna (ataluren), a medicine to treat patients with Duchenne muscular dystrophy whose disease is caused by a type of genetic defect called a ‘nonsense mutation’ in the dystrophin gene and who are able to walk.
For more information on this re-examination opinion, see the updated public health communication included in the grid below.
Outcome of referral
The committee endorsed the measures recommended by EMA’s human medicines safety committee, the PRAC, to minimise the risks of posterior reversible encephalopathy syndrome (PRES) and reversible cerebral vasoconstriction syndrome (RCVS) for medicines containing pseudoephedrine. PRES and RCVS are rare conditions that can involve reduced blood supply to the brain, potentially causing serious, life-threatening complications. With prompt diagnosis and treatment, symptoms of PRES and RCVS usually resolve.
Pseudoephedrine-containing medicines are authorised in various EU Member States alone, or in combination with medicines to treat symptoms of a cold and flu such as headache, fever and pain, or allergic rhinitis (inflammation of the nasal passages) in people with nasal congestion. Pseudoephedrine is also authorised in some EU Member States to treat aerotitis (inflammation of the middle ear due to sudden changes in air pressure) in a fixed-dose combination with triprolidine.
The CHMP opinion will now be sent to the European Commission, which will issue a legally binding decision across the EU.
For more information on this opinion, see the public health communication included in the grid below.
Start of re-examination of recommendations
A group of companies that contracted Synapse Labs Pvt. Ltd to conduct bioequivalent studies for generic medicines has requested a re-examination of EMA’s December 2023 opinion. Upon receipt of the grounds of the request, the Agency will re-examine its opinion and issue a final recommendation.
For more information, see the updated public health communication in the grid below.
Agenda and minutes
The agenda of the January 2024 CHMP meeting is published on EMA’s website. Minutes of the meeting will be published in the coming weeks.
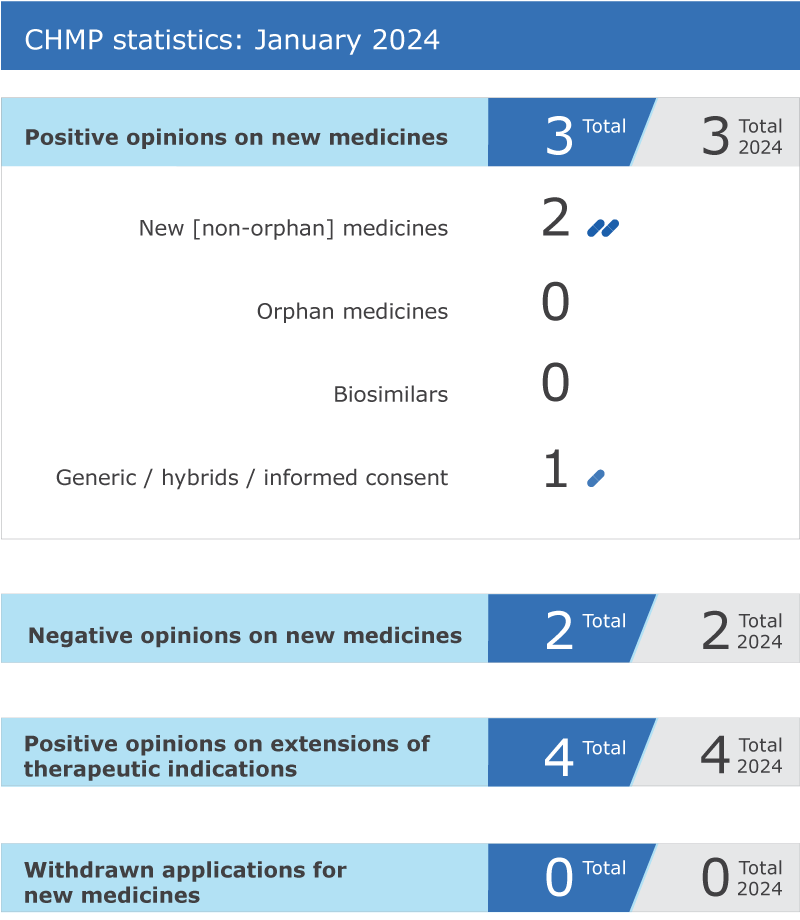
CHMP statistics
Key figures from the January 2024 CHMP meeting are represented in the graphic below.
*This product was designated as an orphan medicine during its development. Orphan designations are reviewed by EMA’s Committee for Orphan Medicinal Products (COMP) at the time of approval to determine whether the information available to date allows maintaining the medicine’s orphan status and granting the medicine ten years of market exclusivity.

