
In a new paper, Sinclair and his co-authors outline a theory arguing that epigenetic changes are the underlying cause of aging [1].
When cells get old
It is not every day that one of the most prominent geroscientists presents a new theory of aging. David Sinclair of Harvard, along with two co-authors, Yuancheng Ryan Lu and Xiao Tian, have just published “The Information Theory of Aging” in Nature Aging. This theory was proposed by Sinclair years ago [2], and this new paper is an attempt to summarize it based on the most recent research.
The ability to store and retrieve information is central to life, which relies on the constant reproduction of complex organisms using DNA blueprints. However, on top of that digital genetic code, there is a much messier realm of epigenetics, which regulates how genetic information is translated into proteins.
There is a vast variety of cells in an organism, all of which have similar genomes. If a cell wants to be, say, a neuron and not a fibroblast, something must tell that cell which genes should be active and to what extent. This something is epigenetic information, and it is stored in a mixed digital-analog format. Epigenetic regulation is performed by numerous mechanisms, such as DNA methylation and acetylation and interfering RNA, and it can be altered by factors like environmental signals and cellular damage.
The information theory of aging postulates that the loss of this regulatory information is the underlying cause of aging that ultimately drives its other manifestations. Simply put, cells accumulate epigenetic noise, gradually becoming worse at what they do. What should have remained inactive starts getting translated and vice versa. This causes cells to become confused, experiencing their own version of Alzheimer’s. Some epigenetic changes are so closely associated with aging that it gave rise to epigenetic clocks, which are among the most reliable aging biomarkers [3].
Reversing the clock
However, epigenetic dysregulation is reversible. The foremost example is what happens very early in an organism’s development. This levels the epigenetic landscape so that a new organism can start from scratch [4]. Unfortunately, we still don’t know how exactly that happens.
DNA mutations have also been proposed as a fundamental cause of aging [5], but Sinclair and his co-authors argue against that. They point out that, unlike epigenetic alterations, DNA mutations cannot be erased: we all carry the mutations accumulated by our ancestors, which is how evolution works. There is other data that points to somatic mutations not being the main driver of aging. For instance, in many experiments, animals cloned from an aged somatic cell with all its accumulated mutations had normal healthy lifespans [6].
A few years ago, scientists learned to mimic this “embryonic reset” to some extent with cellular reprogramming. Using certain genes that control other genes (transcription factors), or even small molecules, we can now erase the identity of aged cells, turning them into induced pluripotent stem cells (iPSCs). This is accompanied by a deep epigenetic rejuvenation.
Later, partial cellular reprogramming was developed [7], in which carefully measured induction of the same factors leads to epigenetic rejuvenation without erasing cellular identity. Both techniques have demonstrated a huge potential in geroscience, extending healthspan and lifespan in various animal models. For instance, we recently covered a paper in which Sinclair’s group showed glaucoma reversal in mice by reprogramming retinal ganglion cells.
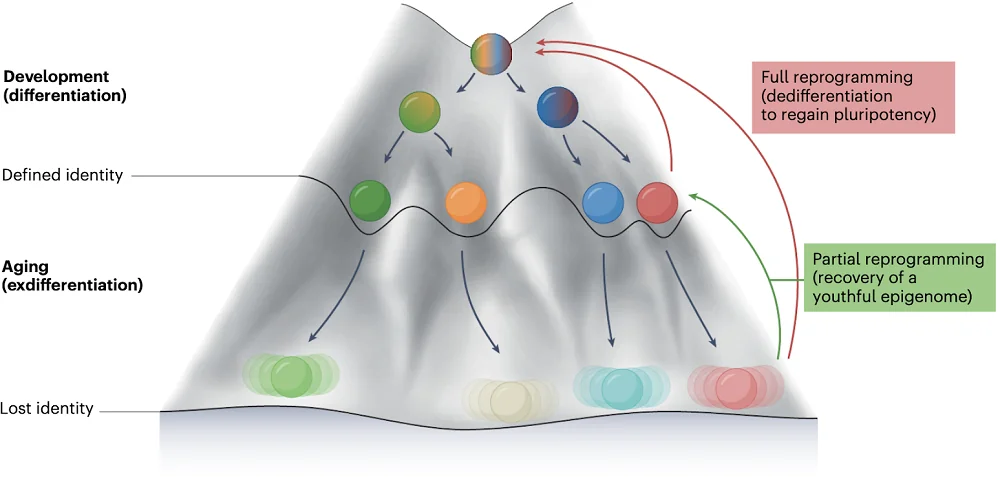
The trade-off
Unfortunately, most living things have evolved in ways that accept the loss of epigenetic information. As scientists have discovered, some proteins of the sirtuin family play a double role, guarding both the genome and the epigenome [8]. When all is calm, those proteins sit still, repressing the transcription of genes that must remain silent in this particular cell type. However, when a double DNA break occurs nearby, they rush to the scene, facilitating the repair. The problem is, they don’t always go back to their stations, which leads to the accumulation of epigenetic noise.
This might be a manifestation of “antagonistic pleiotropy”, in which evolution favors mechanisms that are efficient, such as by serving multiple purposes, but lead to accumulation of damage, which must be just slow enough to allow an organism to reproduce.
Sirtuins’ double role might explain the correlation we observe between the rate of DNA mutations and aging across species. Double-strand breaks may result in DNA mutations and epigenetic alterations both. Both of them are correlated with aging, but only the latter causes it, according to the paper’s authors. In fact, a recent study by Sinclair’s group suggests that DNA breaks cause cellular aging even when faithfully repaired, which is probably due to epigenetic alterations associated with the repair process.
The backup
The brighter part of the information theory of aging is that there must be a “backup copy” of the original, pristine state of the epigenome that cells turn to during an embryonic reset, cellular reprogramming, and probably regeneration events, such an axolotl growing back one of its limbs. Here, the theory borrows a lot from Claude Shannon’s information theory of communication, which is considered foundational for modern communication technologies like the internet and mobile phones.
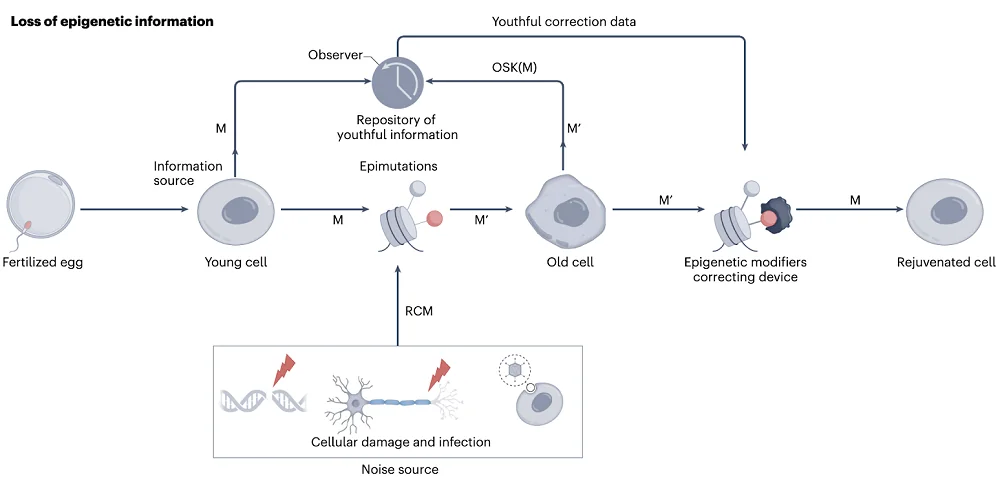
Shannon’s theory involves an observer who has access to both the information at the source and to the transmission as it was received at the destination, and can correct errors, which is exactly how it works in the TCP/IP protocol. Sinclair and his co-authors hope to eventually find a cellular analog of this mechanism and have some ideas about how it might work.
“Passive observers” might be molecules that act as barriers that prevent changing the epigenetic status of a DNA region, be it during aging or by cellular reprogramming mechanisms. The expression of the genes guarded by those molecules remains consistent as time passes. “Active observers” are hypothetical molecules that can mark accumulating epigenetic changes for reprogramming factors to roll them back. Elucidating those mechanisms would be a major leap for geroscience.
Literature
[1] Lu, Y. R., Tian, X., & Sinclair, D. A. (2023). The information theory of aging. Nature Aging, 1-14.
[2] Sinclair, D. A. & LaPlante, M. D. Lifespan: Why We Age—and Why We Don’t Have To (Atria Books, Simon and Schuster, 2019)
[3] Ryan, C. P. (2021). “Epigenetic clocks”: Theory and applications in human biology. American Journal of Human Biology, 33(3), e23488.
[4] Kerepesi, C., Zhang, B., Lee, S. G., Trapp, A., & Gladyshev, V. N. (2021). Epigenetic clocks reveal a rejuvenation event during embryogenesis followed by aging. Science advances, 7(26), eabg6082.
[5] Cagan, A., Baez-Ortega, A., Brzozowska, N., Abascal, F., Coorens, T. H., Sanders, M. A., … & Martincorena, I. (2022). Somatic mutation rates scale with lifespan across mammals. Nature, 604(7906), 517-524.
[6] Burgstaller, J. P., & Brem, G. (2017). Aging of cloned animals: A mini-review. Gerontology, 63(5), 417-425.
[7] Lehmann, M., Canatelli-Mallat, M., Chiavellini, P., Cónsole, G. M., Gallardo, M. D., & Goya, R. G. (2019). Partial reprogramming as an emerging strategy for safe induced cell generation and rejuvenation. Current Gene Therapy, 19(4), 248-254.
[8] Oberdoerffer, P., Michan, S., McVay, M., Mostoslavsky, R., Vann, J., Park, S. K., … & Sinclair, D. A. (2008). SIRT1 redistribution on chromatin promotes genomic stability but alters gene expression during aging. Cell, 135(5), 907-918.




